Bowel-associated dermatosis-arthritis syndrome (BADAS): The story behind the acronym

By Warren R. Heymann, MD, FAAD
March 30, 2022
Vol. 4, No. 13

Intestinal bypass surgery for morbid obesity was first utilized in 1956. Complications such as persistent diarrhea, electrolyte deficits, hepatic failure, renal stones, gallstones, among others were numerous. A 23% incidence of polyarthritis was appreciated, as was tenosynovitis and polymyalgia. Dicken and Seehafer were the first to recognize cutaneous lesions (vesiculopustular lesions in two patients, one of whom also displayed erythema nodosum) following intestinal bypass surgery, suggesting the term “bowel bypass syndrome.” (3) The syndrome demonstrated the histologic features of Sweet syndrome, thereby classifying it as a neutrophilic dermatosis, occurring in 20% of patients who underwent jejunoileal bypass surgery for the treatment of morbid obesity. Treatment with low-dose steroids, tetracycline, or metronidazole suppressed symptoms in most cases, and restoration of normal bowel anatomy was curative. The presumption was that immunologic reactions (the formation of circulating immune complexes) to antigenic bacterial peptidoglycans from numerous intestinal bacteria was pathogenic. (4)
In 1983, Jorizzo et al reported an identical clinicopathologic syndrome in four patients who did not have jejunoileal bypass surgery. Each patient, however, had other gastrointestinal diseases (patients with peptic ulcer disease with a choledochoduodenal fistula, a partial gastrectomy for peptic ulcer disease, Crohn disease, and ulcerative colitis, respectively) that the authors believed predisposed to this syndrome, possibly via circulating immune complexes with bowel-associated antigens. They proposed the expanded term, bowel-associated dermatosis-arthritis syndrome (BADAS), to encompass these new cases. (5)
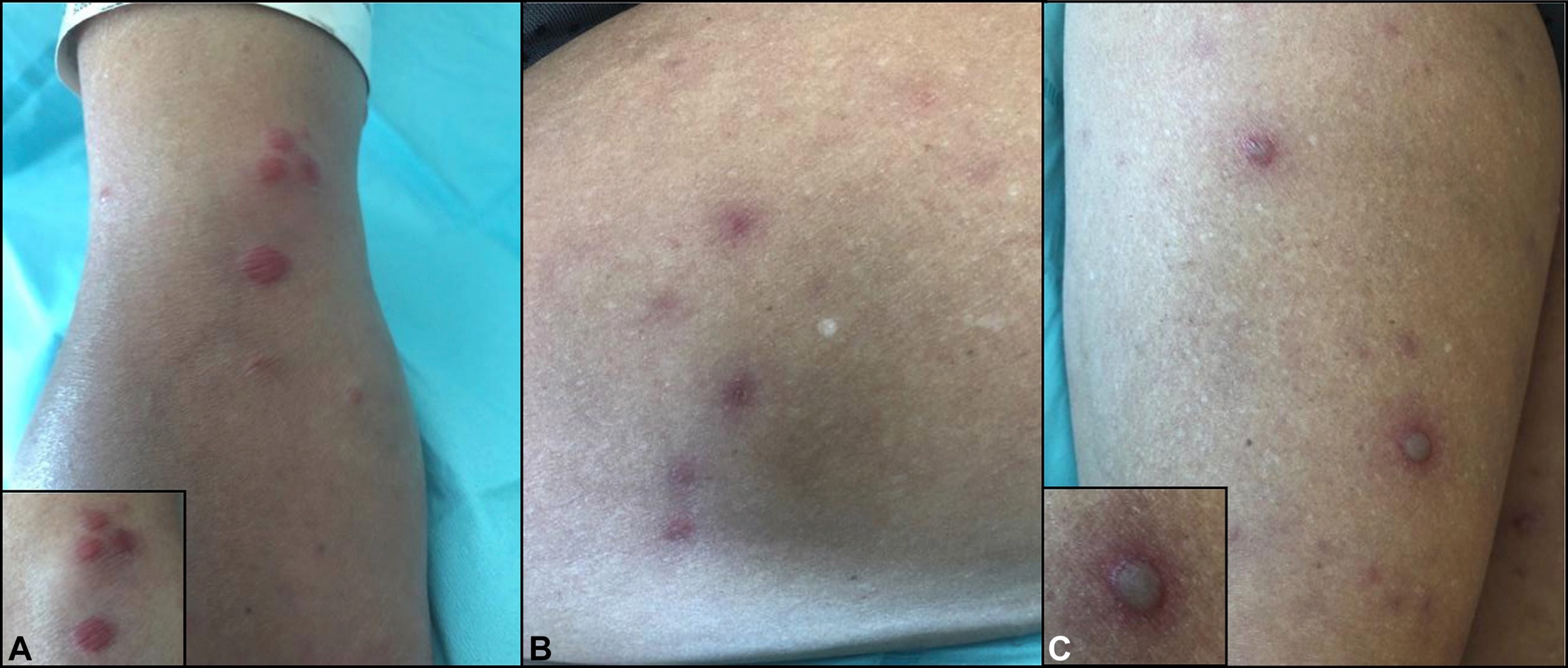
Patton et al provide an excellent summation of BADAS: “Skin lesions classically emerge as erythematous macules 3 to 10 millimeters in diameter and develop into papular, vesicular, and then pustular 2 to 4 millimeter lesions over the subsequent 1 to 2 days. The lesions are sterile and the eruption is usually concentrated on the upper extremities and torso, overall lasting 2 to 8 days with recurrence approximately every 1 to 6 weeks. Skin eruptions may exist in any stage from macule to pustule and may be pruritic, painful, or completely asymptomatic. Lesions may be difficult to distinguish from gonococcal sepsis both clinically and histologically. Arthralgias do not produce radiographic changes or deformities in BADAS. The polyarticular episodic joint pain lasts days to weeks and predominantly affects small peripheral joints such as the fingers and wrists. Polymyalgia with tenosynovitis has been reported. Laboratory evaluation of rheumatoid factor, anti-nuclear antibody, immunoglobulins, and uric acid are usually within normal limits. Cryoglobulins in the serum have been noted in some patients during symptomatic periods.” Histologically, although most cases are identical to Sweet syndrome (a neutrophilic infiltrate without vasculitis), other variations, such as resembling subcorneal pustulosis (Sneddon-Wilkinson) have been reported. (6)
Recent literature on BADAS has expanded some clinical concepts. Although most cases occur in adults, Havele et al reported BADAS in a 6-year-old boy with severe very early-onset inflammatory bowel disease. (7) Rosen et al detailed a case in a 47-year-old woman with cystic fibrosis (CF), proposing that BADAS was due to the high prevalence of small intestinal bacterial overgrowth that may accompany CF. (8) Although most cases of BADAS occur between 3 months and 5 years following gastrointestinal surgery, Richarz et al reported a case in a 54-year-old woman 10 years after her bariatric surgery. (9)
Treatment for BADAS focuses on the pathophysiology of infectious and inflammatory mechanisms. Antibiotics such as tetracycline or metronidazole, steroids (topical and systemic) NSAIDS, and other immunosuppressive agents (cyclosporine, mycophenolate mofetil) have been utilized effectively. (10) Ustekinumab demonstrated significant improvement in a case of BADAS secondary to Crohn proctitis. (11) For BADAS caused by bypass surgery, restoration of normal bowel anatomy, with another surgical operation eliminating the bowel loop, has also been curative in many cases. (10)
Dicken and Seehafer were prescient in their comment: “Perhaps the patients with arthritis, bypass enteropathy, and bowel bypass syndrome represent a spectrum of clinical disease, the severity of which is based on the individual patient’s response to the bacterial products, the immunologic response to these products, or both.” (3) As our knowledge of the intestinal microbiome’s interplay with immunity increases exponentially, we can anticipate that understanding intestinal dysbiosis that has been associated with gastroenterologic, neurologic, respiratory, metabolic, hepatic, and cardiovascular illnesses (12), will also lead to novel approaches for BADAS and other dermatoses such as atopic dermatitis and psoriasis.
Finally, a word about acronyms. It has been argued that non-standardized acronyms contribute to confusion and should be used sparingly (13). I agree — but I have to admit, I love the acronym BADAS.
Point to Remember: The bowel-associated dermatosis-arthritis syndrome is a neutrophilic dermatosis that may be seen in patients following bariatric surgery and those with underlying gastrointestinal disorders.
Our expert’s viewpoint
Joseph L. Jorizzo, MD, FAAD
Professor and Former & Founding Chair, Department of Dermatology
Wake Forest University School of Medicine
Clinical Professor Department of Dermatology
Weill Cornell Medical College
In 1983 the fascinating dermato-rheumatologic syndrome known as jejunoileal bypass syndrome was about to be relegated to the history books due to the abandonment of that surgical procedure. Instead, it provided an opportunity to learn from the purported pathogenesis of that syndrome and gain important insights into reactive dermatoses associated with labile inflammatory bowel disease including bowel-associated dermatosis arthritis syndrome. The designation BADAS resulted from an initial obsession with substituting the dermatologically correct term, dermatosis, for the term “dermatitis” (preferred by internists), which is a synonym for eczema. Only later did the amusing aspect of the BADAS designation become obvious. Ultimately the idea that reactive dermatoses characterized by a histopathology dominated by dermal neutrophilic infiltrates overlapped with BADAS led to the concept of neutrophilic dermatoses. (14, 15)
https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed August 7, 2021)
Staudenmann DA, Sui Z, Saxena P, Kaffes AJ, Marinos G, Kumbhari V, Aepli P, Sartoretto A. Endoscopic bariatric therapies for obesity: a review. Med J Aust. 2021 Aug 1. doi: 10.5694/mja2.51179. Epub ahead of print. PMID: 34333788.
Dicken CH, Seehafer JR. Bowel bypass syndrome. Arch Dermatol. 1979 Jul;115(7):837-9. PMID: 453891.
Ely PH. The bowel bypass syndrome: a response to bacterial peptidoglycans. J Am Acad Dermatol. 1980 Jun;2(6):473-87. doi: 10.1016/s0190-9622(80)80148-4. PMID: 7400404.
Jorizzo JL, Apisarnthanarax P, Subrt P, Hebert AA, Henry JC, Raimer SS, Dinehart SM, Reinarz JA. Bowel-bypass syndrome without bowel bypass. Bowel-associated dermatosis-arthritis syndrome. Arch Intern Med. 1983 Mar;143(3):457-61. PMID: 6830382.
Patton T, Jukic D, Juhas E. Atypical histopathology in bowel-associated dermatosis-arthritis syndrome: A case report. Dermatol Online J. 2009 Mar 15;15(3):3. PMID: 19379647.
Havele SA, Clark AK, Oboite M, Conrad MA, Perman MJ, Rubin AI, Treat JR. Bowel-associated dermatosis-arthritis syndrome in a child with very early onset inflammatory bowel disease. Pediatr Dermatol. 2021 May;38(3):697-698. doi: 10.1111/pde.14544. Epub 2021 Mar 21. PMID: 33749007.
Rosen JD, Stojadinovic O, McBride JD, Rico R, Cho-Vega JH, Nichols AJ. Bowel-associated dermatosis-arthritis syndrome (BADAS) in a patient with cystic fibrosis. JAAD Case Rep. 2018 Dec 5;5(1):37-39. doi: 10.1016/j.jdcr.2018.08.029. PMID: 30581933; PMCID: PMC6287091.
Richarz NA, Bielsa I, Morillas V, Enguita V, Fumagalli C. Bowel-associated dermatosis-arthritis syndrome (BADAS). Australas J Dermatol. 2021 May;62(2):241-242. doi: 10.1111/ajd.13516. Epub 2020 Dec 12. PMID: 33314024.
Hassold N, Jelin G, Palazzo E, Deschamps L, Forien M, Ottaviani S, Descamp V, Dieudé P. Bowel-associated dermatosis arthritis syndrome: A case report with first positron emission tomography analysis. JAAD Case Rep. 2019 Jan 25;5(2):140-143. doi: 10.1016/j.jdcr.2018.10.025. PMID: 30733980; PMCID: PMC6355321.
Heard M, Zhang M, Jorizzo JL. A case of bowel-associated dermatosis-arthritis syndrome treated with ustekinumab: The importance of targeting underlying gastrointestinal disease. JAAD Case Rep. 2020 May 5;6(6):506-508. doi: 10.1016/j.jdcr.2020.04.001. PMID: 32490110; PMCID: PMC7256244.
Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016 Dec 15;375(24):2369-2379. doi: 10.1056/NEJMra1600266. PMID: 27974040.
Hon KL, Leung AKC, Wong JCP. Proliferation of syndromes and acronyms in paediatric critical care: are we more or less confused? Hong Kong Med J. 2020 Jun;26(3):260-262. doi: 10.12809/hkmj198059. PMID: 32554820.
Jorizzo JL, Solomon AR, Zanolli MD, Leshin B. Neutrophilic vascular reactions. J Am Acad Dermatol. 1988 Dec;19(6):983-1005. doi: 10.1016/s0190-9622(88)70264-9. PMID: 3060489.
Alavi A, Sajic D, Cerci FB, Ghazarian D, Rosenbach M, Jorizzo J. Neutrophilic dermatoses: an update. Am J Clin Dermatol. 2014 Oct;15(5):413-23. doi: 10.1007/s40257-014-0092-6. PMID: 25154386.
All content found on Dermatology World Insights and Inquiries, including: text, images, video, audio, or other formats, were created for informational purposes only. The content represents the opinions of the authors and should not be interpreted as the official AAD position on any topic addressed. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment.
DW Insights and Inquiries archive
Explore hundreds of Dermatology World Insights and Inquiries articles by clinical area, specific condition, or medical journal source.
All content solely developed by the American Academy of Dermatology
The American Academy of Dermatology gratefully acknowledges the support from Incyte Dermatology.
 Make it easy for patients to find you.
Make it easy for patients to find you.
 Meet the new AAD
Meet the new AAD
 2022 AAD VMX
2022 AAD VMX
 AAD Learning Center
AAD Learning Center
 Need coding help?
Need coding help?
 Reduce burdens
Reduce burdens
 Clinical guidelines
Clinical guidelines
 Why use AAD measures?
Why use AAD measures?
 Latest news
Latest news
 New insights
New insights
 Combat burnout
Combat burnout
 Joining or selling a practice?
Joining or selling a practice?
 Advocacy priorities
Advocacy priorities
 Promote the specialty
Promote the specialty

