'Stepping up' to off-label dosing of biologics

By David A. Wetter, MD, FAAD
April 6, 2022
Vol. 4, No. 14

A patient in his forties with chronic generalized plaque psoriasis recently contacted me regarding his adalimumab prescription. His disease had been well-controlled with a dosage of 40 milligrams (mg) every 2 weeks for six months, but he had noticed that his psoriasis started to recur a few days before his next scheduled adalimumab treatment. The patient wondered if he would benefit by increasing the frequency of his adalimumab treatment to every week.
A similar patient with chronic generalized plaque psoriasis, also in his forties, whose disease had been in remission for several years with ustekinumab at a dosage of 90 mg every 12 weeks observed that his psoriasis was now under 85%-90% control; he wondered how to recapture his complete response. Given that the patient had no side effects and was still experiencing good results from his treatment, could we increase the frequency of his ustekinumab treatment to every 8 weeks, instead of switching to an alternative medication?
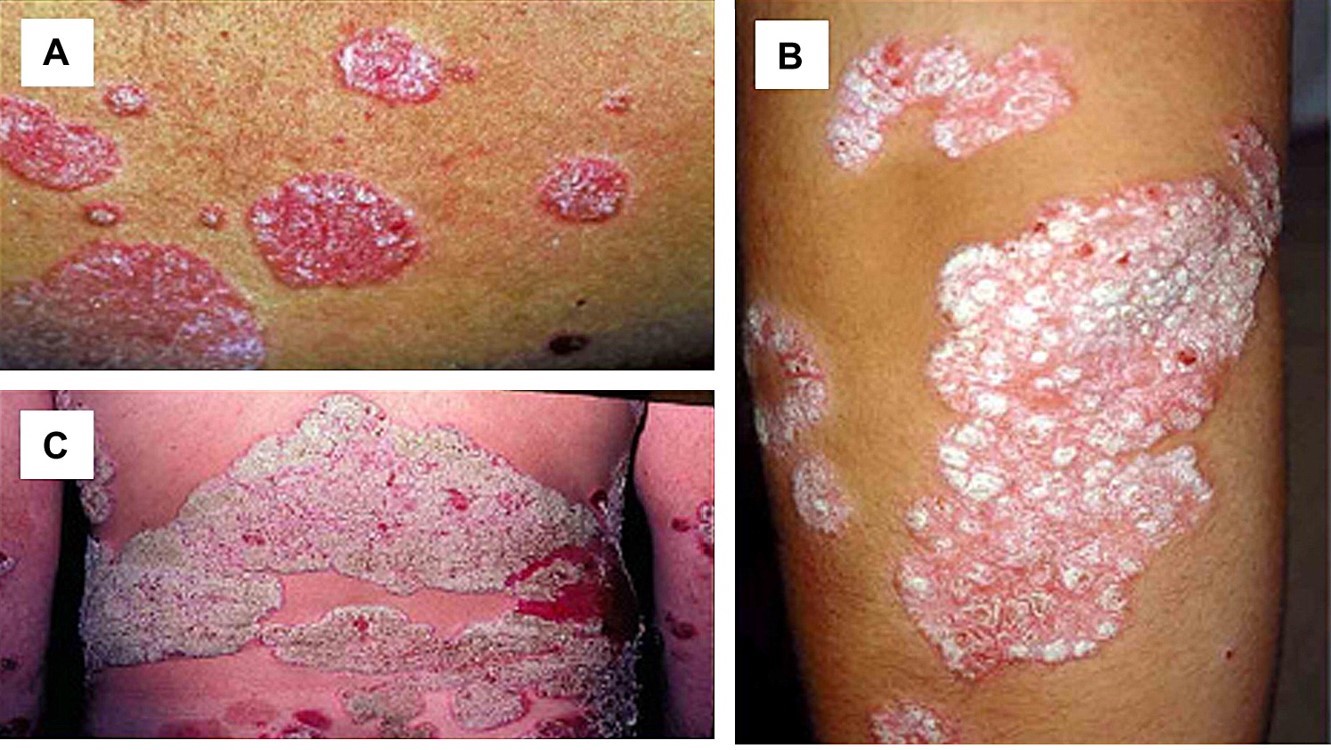
These patient encounters spurred me to seek out guidance from the medical literature, and a few of the findings are shared below:
A systematic review of dose adjustment of biologic treatments for psoriasis in the “real-world” setting found that dose escalation (i.e. shortening the dosing interval, or increasing the administered dose) commonly occurred, particularly for tumor necrosis factor-alpha (TNF-alpha) inhibitors (e.g. etanercept, adalimumab, infliximab) and ustekinumab. (3)
The British Association of Dermatologists’ guidelines for biologic treatment for psoriasis recommend to “consider escalating the dose of or reducing the interval for biologic therapy in adults and when an inadequate primary response might be due to insufficient drug exposure (e.g., in people who are obese and/or whose psoriasis relapses during the treatment cycle and/or if the drug level is known to be subtherapeutic).” (2) Specific dose-escalation and interval-reduction strategies enumerated in the guidelines include: adalimumab 40 mg weekly; etanercept 50 mg twice weekly; ustekinumab 90 mg every 8 or 12 weeks (if ≤ 100 kg) and 90 mg every 8 weeks (if > 100 kg); ixekizumab 80 mg every 2 weeks; and tildrakizumab 200 mg every 12 weeks (if high disease burden or ≥ 90 kg).
Analysis of data from an open-label study of adult patients with moderate-to-severe psoriasis who had not achieved an adequate response after 24 weeks of receiving adalimumab 40 mg every other week demonstrated clinical improvement (without additional adverse effects) with an increased adalimumab dosage of 40 mg every week. (4)
Dose escalation strategies for biologic treatments also have been successfully reported for other dermatologic diseases, including atopic dermatitis and hidradenitis suppurativa:
In an observational prospective cohort study of 221 patients with atopic dermatitis who were treated with dupilumab 300 mg every two weeks, 12 patients had their dosing interval shortened to either every 7 days (eight patients) or every 10 days (four patients). (5) Clinical improvement was noted in 6 of the 7 patients for whom adequate follow-up time was available.
A retrospective case series of 14 patients with hidradenitis suppurativa whose disease either had an initial inadequate response to, or partial loss of initial response to, adalimumab 40 mg weekly (FDA-labeled dosing for hidradenitis suppurativa) demonstrated enhanced clinical response when adalimumab was increased to 80 mg weekly. (6)
In contrast to FDA-labeled maintenance dosage for psoriasis of infliximab 5 mg/kg every 8 weeks, both a prospective analysis of 42 patients (7) and a retrospective cohort study of 52 patients (8) found that a higher dose and frequency of infliximab (up to 10 mg/kg every 4 weeks) is needed to achieve optimal clinical response for patients with hidradenitis suppurativa.
Although off-label dosing of biologic treatments for medical dermatologic diseases is being increasingly reported in the literature, authors caution that because of the higher annual economic costs of treatment associated with off-label escalation dosing, prescriptions may not be readily approved by insurance companies. (2, 3) Emblematic of this challenge, a recent study of patients with complex medical dermatologic disease seen within an academic rheumatology-dermatology clinic found that prior authorizations of off-label systemic medications were commonly denied and led to delays in patient treatment as well as sizable administrative burden for dermatologic practices. (9)
What were the outcomes for my two patients described at the beginning of this commentary? For the first patient, we increased his adalimumab dosage to 40 mg every week, which led to complete response of his psoriasis without side effects (and insurance continued to provide coverage for the off-label dosing). For the second patient, we shortened the dosing interval of ustekinumab (90 mg) to every 8 weeks. Unfortunately, despite a detailed letter of medical necessity with an accompanying medical literature reference, his insurance company denied the request for off-label dosing and the patient preferred to switch to an alternative biologic treatment rather than “continue to fight with the insurance company."
Point to Remember: “Off-label” dosing (i.e. higher dose or reduced interval between doses) of biologic treatments for patients with complex medical dermatologic diseases (including psoriasis, atopic dermatitis, and hidradenitis suppurativa) may be a helpful strategy to counteract initial suboptimal disease control or disease recurrence that occurs shortly before the next scheduled biologic dose. Hopefully dermatologists will feel empowered to “step up to the plate” and utilize off-label biologic dosing when indicated for their patients!
Our editor’s viewpoint
Warren Heymann, MD, FAAD
I would wager that every dermatologist uses “off-label” prescriptions on a daily basis. For example, every time a calcineurin inhibitor is prescribed for any disorder other than atopic dermatitis, it is “off-label.” Because of the expense of biologics, “off-label” prescribing is an important issue as detailed in Dr. Wetter’s excellent commentary. For the scenarios he described, I have usually been successful, after jumping through hoops and facing denials, until I am able to have reasonable “one-on-one” discussion with a sympathetic medical director. Dr. Wetter is correct in reminding us to “step-up” as our patients’ advocate. Unfortunately, that phrase brings “step therapy” to mind, which, in my estimation is an abysmal failure of our current system. An example is using methotrexate or cyclosporine before being able to get dupilumab approved for atopic dermatitis. It is a moral violation to utilize drugs that are potentially more toxic and less efficacious before getting to the optimal drug. As we move forward with health care reform, both easing “off-label” prescribing and elimination of dangerous step therapies must be a priority.
Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
Smith CH, Yiu ZZN, Bale T, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2020: A rapid update. Br J Dermatol. 2020;183:628-637.
Gambardella A, Licata G, Sohrt A. Dose adjustment of biologic treatments for moderate-to-severe plaque psoriasis in the real world: A systematic review. Dermatol Ther (Heidelb). 2021;11:1141-1156.
Gniadecki R, Leonardi CL, Gordon KB, et al. Long-term optimization of outcomes with flexible adalimumab dosing in patients with moderate to severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1297-1304.
Bosma AL, de Wijs LEM, Hof MH, et al. Long-term effectiveness and safety of treatment with dupilumab in patients with atopic dermatitis: Results of the TREAT NL (TREatment of Atopic eczema, the Netherlands) registry. J Am Acad Dermatol. 2020;83:1375-1384.
Zouboulis CC, Hansen H, Caposiena Caro RD, et al. Adalimumab dose intensification in recalcitrant hidradenitis suppurativa/acne inversa. Dermatology. 2020;236:25-30.
Ghias MH, Johnston AD, Kutner AJ, et al. High-dose, high-frequency infliximab: A novel treatment paradigm for hidradenitis suppurativa. J Am Acad Dermatol. 2020;82:1094-1101.
Oskardmay AN, Miles A, Sayed CJ. Determining the optimal dose of infliximab for treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;81:702-708.
Jew OS, Okawa J, Barbieri JS, et al. Evaluating the effect of prior authorizations in patients with complex dermatologic conditions. J Am Acad Dermatol. 2020;83:1674-1680.
All content found on Dermatology World Insights and Inquiries, including: text, images, video, audio, or other formats, were created for informational purposes only. The content represents the opinions of the authors and should not be interpreted as the official AAD position on any topic addressed. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment.
DW Insights and Inquiries archive
Explore hundreds of Dermatology World Insights and Inquiries articles by clinical area, specific condition, or medical journal source.
All content solely developed by the American Academy of Dermatology
The American Academy of Dermatology gratefully acknowledges the support from Incyte Dermatology.
 Make it easy for patients to find you.
Make it easy for patients to find you.
 Meet the new AAD
Meet the new AAD
 2022 AAD VMX
2022 AAD VMX
 AAD Learning Center
AAD Learning Center
 Need coding help?
Need coding help?
 Reduce burdens
Reduce burdens
 Clinical guidelines
Clinical guidelines
 Why use AAD measures?
Why use AAD measures?
 Latest news
Latest news
 New insights
New insights
 Combat burnout
Combat burnout
 Joining or selling a practice?
Joining or selling a practice?
 Advocacy priorities
Advocacy priorities
 Promote the specialty
Promote the specialty

