Eye-opening news about oxymetazoline

By Warren R. Heymann, MD, FAAD
April 27, 2022
Vol. 4, No. 17

Oxymetazoline is a potent vasoconstrictor that also demonstrates anti-inflammatory properties. It is a direct-acting, imidazoline-type sympathomimetic agonist that is highly selective for the α1A-adrenoceptor and is also a partially selective α2A-receptor agonist. (1) The drug’s vasoconstrictive effects have been used to advantage for other dermatological conditions, including post-acne erythema (3,4), erythromelalgia of the knee (5), and hemostasis during dermatologic surgery. (6)
Oxymetazoline 0.1% ophthalmic solution is now available for the treatment of blepharoptosis (drooping of the eyelid, subsequently referred to as ptosis). This commentary will focus on the use of oxymetazoline for ptosis. (I have no financial or any other perceived conflict of interest with RVL Pharmaceuticals, Inc. the distributor of Upneeq.)
Ptosis may be a cosmetic concern but if significant enough, may interfere with vision. I have mild ptosis — people have told me to “wake up” during our conversations. (Sometimes they are correct and I am falling asleep!)
Ptosis is a physical sign, not a diagnosis. Ptosis may be congenital or acquired. Acquired adult ptosis is categorized as 1) aponeurotic (aka involutional; this is the most common form, usually presenting in the 5th-6th decade, most frequently due to dehiscence or disinsertion of the levator aponeurosis; 2) neurogenic (due to disruption of innervation of either the levator muscle or Muller muscle [a small smooth muscle arising from the striated levator muscle along with the aponeurosis] as may be seen with Horner syndrome or 3rd cranial nerve palsy; 3) myogenic (due to an abnormality of levator muscle itself, as seen with myasthenia gravis or myotonic dystrophy; 4) mechanical (secondary to any tumor causing increased eyelid weight, conjunctival scarring, and blepharochalasis); and 5) traumatic (due to direct or indirect injury to the levator muscle). (7) If there is any question about the etiology of ptosis an ophthalmological consultation should be sought.
Oxymetazoline elevates the eyelid by activating α-adrenergic receptors in the Muller muscle. Slonim et al reported the results of 2 industry-sponsored randomized placebo-controlled masked trials that assessed the efficacy of oxymetazoline, 0.1%, eyedrops for ptosis. A total of 304 patients with acquired ptosis to topical oxymetazoline HCl, 0.1%, vs vehicle in 2 trials were randomized. All participants had clinically relevant involutional (aponeurotic) ptosis defined by superior visual field loss and reduced eyelid height. Response to therapy was evaluated at 1 day and 14 days of treatment, and safety was evaluated over 42 days of follow-up. Using Leicester Humphrey Visual Field (HVF) static perimetry as the primary outcome, the authors showed a change in the oxymetazoline treatment arm of 4 to 5 points of superior visual field more than the change in the vehicle arm. The secondary outcome, change in upper eyelid height, increased by less than 0.5 mm in the treatment arm compared with the vehicle arm on day 1 and by 0.67 mm compared with vehicle on day 14.
Oxymetazoline HCl, 0.1%, was generally well tolerated, with no change in visual acuity or intraocular pressure but with somewhat higher rates of punctate keratitis, conjunctival hyperemia, and subjectively blurred vision in the study group. (8,9) In an editorial accompanying the article by Slonim at al, Bradley and Bradly opine; “It is unclear whether a gain of 4 to 5 points of superior visual field, or roughly half a line of points on the Leicester HVF, represents meaningful clinical change. The corresponding less than 1 mm of eyelid elevation produced by oxymetazoline compared with vehicle may also be of marginal clinical benefit. While it is possible that this degree of eyelid elevation yields substantial improvements in visual function and patient self-image, an assessment of health-related quality of life was not included in the study.” Additionally, this study did not compare oxymetazoline with surgery, although when compared to historical data, the improvement with oxymetazoline is less than is usually observed with surgical repair. (9)
Wirta et al (with Slonim as senior author) evaluated the safety profile of oxymetazoline 0.1% when administered once daily for 14-84 days to assess treatment-emergent adverse events (TEAE). A total of 568 participants with acquired blepharoptosis were evaluated. The median age was 66 years and 74.8% of participants were female. Overall, 375 participants self-administered oxymetazoline 0.1% to both eyes once/day and 193 self-administered placebo (vehicle) daily. TEAE incidence was similar among participants using oxymetazoline 0.1% (31.2%) or vehicle (30.6%). Nearly all TEAEs were mild-to-moderate, and most were not suspected of being treatment related. Serious TEAEs occurred in four participants receiving oxymetazoline 0.1% and one participant receiving vehicle. Nine and 2 participants in the oxymetazoline 0.1% and vehicle groups, respectively, discontinued due to a TEAE. Ocular TEAEs occurring in ≥2% of participants receiving oxymetazoline 0.1% were punctate keratitis, conjunctival hyperemia, dry eye, blurred vision, instillation site pain, and corneal vital dye staining, with none occurring in >3.5% of participants, allowing the authors to conclude that oxymetazoline for ptosis is safe and well tolerated.
Wirta et al explain that oxymetazoline may affect α-adrenergic receptors expressed elsewhere in the eye (conjunctiva, iris-ciliary structures, aqueous outflow tract) that could explain the adverse reactions of punctate keratitis, conjunctival hyperemia, and dry eye. Finally, oxymetazoline may be associated with transient mydriasis and acute angle closure glaucoma – trials with oxymetazoline excluded such patients. (10)
Dermatologists may encounter ptosis due to localized spread of botulinum toxin when administered for treating glabellar lines. Oxymetazoline may be considered as convenient, rapid, safe, and effective treatment for this adverse reaction to botulinum toxin. (11)
Before prescribing oxymetazoline, I would encourage all to read the package insert. Avoid using the medication in those with a history of acute angle glaucoma and be judicious with its use in hypertensive patients.
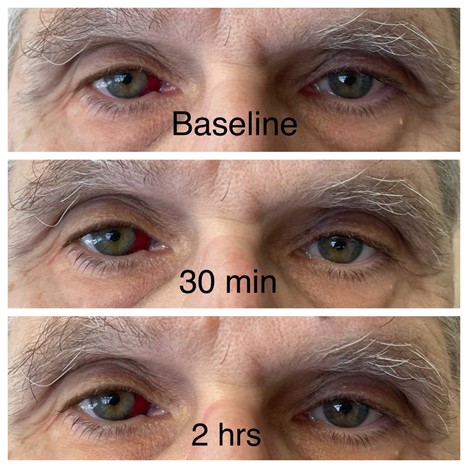
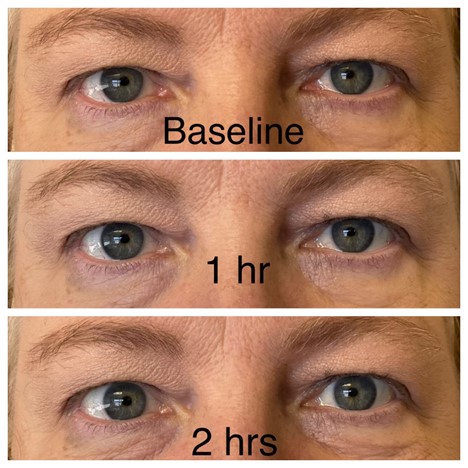
I could not resist the temptation. I tried it, but my results were barely perceptible (see below, and please ignore the subconjunctival hemorrhage). Our practice manager, however, was delighted with her results that were observed within an hour (see below, with permission).
Acknowledgement: I thank Lacy L. Sommer, MD, FAAD, for her photographic skills.
Warren R. Heymann, MD, FAAD
Practice manager
Point to Remember: Oxymetazoline has been used increasingly and successfully for its vasoconstrictive properties in rosacea, erythromlelagia, and post-acne erythema. The α-adrenergic receptors in the Muller muscle allow the drug to benefit patients with ptosis.
Our expert’s viewpoint
Mark S. Nestor, MD, PhD, FAAD
Director, Center for Cosmetic Enhancement
Director, Center for Clinical and Cosmetic Research
Aventura, FL
Voluntary Professor
Department of Dermatology and Cutaneous Surgery
Department of Surgery, Division of Plastic Surgery
University of Miami Miller School of Medicine
Miami, FL
While uncommon and ultimately self-limiting, botulinum toxin A induced blepharoptosis can cause distress both for the patient and for the treating physician. It occurs due to a toxin-induced weakness of the levator palpebrae superioris muscle. It normally is seen between three and 14 days after injection and normally resolves as the effect of the toxin wanes. While it is most commonly caused by inappropriate injection in high-risk zones it also can occur based on anatomical differences in some patients whereby a supraorbital foramen maybe present which causes a shortcut from the brow area to the muscle. Risk definitely decreases with experience and thorough anatomic knowledge of risk areas. A new treatment option (Oxymetazoline HCL 0.1%, Upneeq, RVL Pharmaceuticals) is now available which can significantly reduce the intensity and duration of the induced ptosis. (11)
Dr. Nestor had disclosed financial relationships with the following to the AAD at the time of publication: Aerolase, Allergan, Inc, Arcutis Biotherapeutics, Biofrontera AG, Bioregenerative Aesthetics, Inc., BirchBioMed, Brickell Biotech, Inc., Cara Therapeutics, Castle Biosciences, Inc, Croma-Pharma, Dermata Therapeutics, DermTech Inc., DUSA Pharmaceuticals, Eirion Therapeutics, Fastox Pharma, Ferndale Laboratories, Inc., Galderma Laboratories, LP, GmbH Austria, Ipsen, Kimera Labs, Inc, Krystal Biotech, Inc, MediWound, Merz Pharmaceuticals, LLC, Novaestiq Corp, Pulse Biosciences, Revian LTD., Rohrer Aesthetics, Seaspire Skincare, Sensus Healthcare, Sirnaomics, Inc., SkinJect Inc., Strathspey Crown, Suneva Medical, Inc., Vial. Full disclosure information is available at coi.aad.org.
Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007 Nov;143(11):1369-71. doi: 10.1001/archderm.143.11.1369. PMID: 18025359.
Okwundu N, Cline A, Feldman SR. Difference in vasoconstrictors: oxymetazoline vs. brimonidine. J Dermatolog Treat. 2021 Mar;32(2):137-143. doi: 10.1080/09546634.2019.1639606. Epub 2019 Aug 12. PMID: 31294643.
Kalantari Y, Dadkhahfar S, Etesami I. Post-acne erythema treatment: A systematic review of the literature. J Cosmet Dermatol. 2022 Apr;21(4):1379-1392. doi: 10.1111/jocd.14804. Epub 2022 Feb 3. PMID: 35076997.
Agamia N, Essawy M, Kassem A. Successful treatment of the face post acne erythema using a topically applied selective alpha 1-Adrenergic receptor agonist, oxymetazoline 1.5%, a controlled left to right face comparative trial. J Dermatolog Treat. 2022 Mar;33(2):904-909. doi: 10.1080/09546634.2020.1789045. Epub 2020 Jul 7. PMID: 32602755.
Altemir A, Iglesias-Sancho M, Barrabés-Torrella C, Sanchez-Regaña M, Salleras-Redonnet M. Successful treatment of knee erythromelalgia with topical oxymetazoline. Clin Exp Dermatol. 2022 Apr;47(4):778-780. doi: 10.1111/ced.15060. Epub 2022 Jan 4. PMID: 34905256.
Barklund JS, Wong EB, Brown M. Use of Oxymetazoline Hydrochloride 0.05% Soaked Pledgets for Hemostasis of Exposed Nasal Mucosa After Dermatologic Surgery. Dermatol Surg. 2021 Feb 1;47(2):305-306. doi: 10.1097/DSS.0000000000002272. PMID: 31809355.
Koka K, Patel BC. Ptosis Correction. 2021 Nov 2. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 30969650.
Slonim CB, Foster S, Jaros M, Kannarr SR, Korenfeld MS, Smyth-Medina R, Wirta DL. Association of Oxymetazoline Hydrochloride, 0.1%, Solution Administration With Visual Field in Acquired Ptosis: A Pooled Analysis of 2 Randomized Clinical Trials. JAMA Ophthalmol. 2020 Nov 1;138(11):1168-1175. doi: 10.1001/jamaophthalmol.2020.3812. PMID: 33001144; PMCID: PMC7530825.
Bradley EA, Bradley DJ. Oxymetazoline for Ptosis. JAMA Ophthalmol. 2020 Nov 1;138(11):1176-1177. doi: 10.1001/jamaophthalmol.2020.3833. PMID: 33001145.
Wirta DL, Korenfeld MS, Foster S, Smyth-Medina R, Bacharach J, Kannarr SR, Jaros MJ, Slonim CB. Safety of Once-Daily Oxymetazoline HCl Ophthalmic Solution, 0.1% in Patients with Acquired Blepharoptosis: Results from Four Randomized, Double-Masked Clinical Trials. Clin Ophthalmol. 2021 Oct 8;15:4035-4048. doi: 10.2147/OPTH.S322326. PMID: 34675472; PMCID: PMC8517985.
Nestor MS, Han H, Gade A, Fischer D, Saban Y, Polselli R. Botulinum toxin-induced blepharoptosis: Anatomy, etiology, prevention, and therapeutic options. J Cosmet Dermatol. 2021 Oct;20(10):3133-3146. doi: 10.1111/jocd.14361. Epub 2021 Aug 11. PMID: 34378298.
All content found on Dermatology World Insights and Inquiries, including: text, images, video, audio, or other formats, were created for informational purposes only. The content represents the opinions of the authors and should not be interpreted as the official AAD position on any topic addressed. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment.
DW Insights and Inquiries archive
Explore hundreds of Dermatology World Insights and Inquiries articles by clinical area, specific condition, or medical journal source.
All content solely developed by the American Academy of Dermatology
The American Academy of Dermatology gratefully acknowledges the support from Incyte Dermatology.
 Make it easy for patients to find you.
Make it easy for patients to find you.
 Meet the new AAD
Meet the new AAD
 2022 AAD VMX
2022 AAD VMX
 AAD Learning Center
AAD Learning Center
 Need coding help?
Need coding help?
 Reduce burdens
Reduce burdens
 Clinical guidelines
Clinical guidelines
 Why use AAD measures?
Why use AAD measures?
 Latest news
Latest news
 New insights
New insights
 Combat burnout
Combat burnout
 Joining or selling a practice?
Joining or selling a practice?
 Advocacy priorities
Advocacy priorities
 Promote the specialty
Promote the specialty

