One Toker over the line: Clarifying Toker cell hyperplasia in Zuska disease and its relationship to hidradenitis suppurativa

By Warren R. Heymann, MD, FAAD
April 20, 2022
Vol. 4, No. 16

ZD (aka mammillary fistula [MF]) was first reported by Zuska et al in 1951 who described a case series of 5 women (with his wife as the index patient) in what were previously considered nontuberculous breast fistulae. The presumption was that lactiferous ducts became obstructed by squamous metaplasia, resulting in subareolar breast abscesses. The mean age of presentation of ZD is 47 years and smoking is strongly associated with the disease. A rare complication of ZD is squamous cell carcinoma of the breast. (2,3)
Cosman and Al-Refaie were the first to recognize extramammary cutaneous findings in ZD, reporting on 2 women in their forties, with evidence of hidradenitis suppurativa (HS) in the inguinal and axillary regions, respectively. The following are the authors’ conclusions: “We present two patients with MF and concurrent acne inversa/HS lesions. These cases raise the question of whether MF might be a manifestation of acne inversa. Despite the ample literature on MF and the very extensive literature on HS, this is a previously unrecognized association, suggesting either that these cases are unusual or that they demonstrate something that has not been seen because it has not been looked for. In its lack of description of associated conditions, the literature contains the implicit assumption that MF is an isolated lesion of the breast. Our cases challenge that assumption, and it remains for surgeons who see MF frequently to confirm or deny the suggested association with acne inversa/HS.” (4)
Toker cells (TC) are normal components of skin of the nipple and areola, thought to be derived from epidermally located mammary ductal epithelium. TC are observed in approximately 10% of nipple sections with routine stains and up to 90% of nipple specimens by CK7 staining, with greater numbers of cells located around nipple orifices. TC may be seen as isolated cells, in small clusters, or as gland-like structures. TCH refers to cases in which TC are found in increased numbers (defined as up to 20 single cells or up to 10 glands). The practical issue is in differentiating TCH from it mimic — Paget disease (PD) of the nipple. Paget cells tend to be more pleomorpbic and mitotic figures may be observed, compared to TC which, at most, have mild cytological atypia. In problematic cases, histochemical stains for mucin or immunostains may be needed to differentiate the two. (Paget cells stain with mucin stains such as mucicarmine, while TC are negative. Immunohistochemically, TC stain for CK7, CAM5.2, and EMA; variable staining is seen for ER and PR, and do not show significant staining for HER2, CK20, GCDFP-15, S100 protein, CD138, p53,p63, or CEA. Paget cells similarly stain with CK7, CAM5.2 and EMA and also show variable staining for ER and PR. Most importantly, the vast majority of cases of Paget disease are strongly HER2 positive; this may be considered the most helpful immunostain differentiating PD from TCH.) (5)
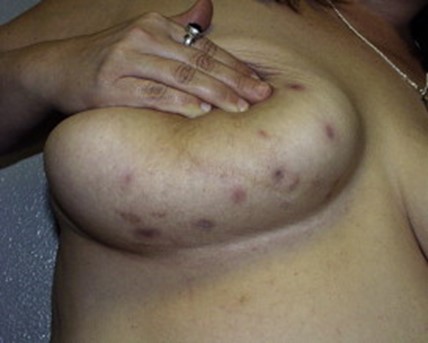
Torre-Castro presented the case of a 42 year-old smoker with recurrent painful inflammatory lesions of her nipples, presenting as nipple inversion. Biopsies were performed because of the clinical concern for PD. The presence of large pale cells was initially thought to be consistent with PD — further differentiating immunohistochemical stains were performed confirming the correct diagnosis of TCH when the patient provided additional history of having similar inflammatory lesions of her groin and having a sister with HS. (1)
Squamous cell carcinoma (SCC) may be considered the most severe complication of HS — I am still haunted by my examination of a woman I encountered as a first-year dermatology resident. She had a fungating, filiform anal lesion, emanating from her scarred perianal HS, that tracked up to her cervical spine, ultimately causing her demise. The prevalence of SCC associated with HS is approximately 4.6%* and is more common among men. (*SCC complicating HS is considered rare — the reported prevalence seems high in my estimation.) As in other scars that predispose to the development of SCC, presumably chronic irritation and inflammation of HS drive the malignant transformation to SCC; additionally, other potential risk factors for malignant transformation may include human papillomavirus (HPV) infection and tobacco use. (6) I surmise that the cases of SCC of the breast in ZD are essentially the same process.
In my PubMed search (performed Jan. 1, 2021) I cannot find any cases of PD in patients with ZD or HS. Of course, anything can happen. Torre-Castro et al have done dermatologists a service by pointing out how we need to be cognizant of TCH, so patients do not get misdiagnosed as PD in the context of ZD/HS. Such patients have enough to deal with already.
Point to Remember: In all likelihood, Zuska disease is a localized form of hidradenitis suppurativa. Although this may rarely be complicated by squamous cell carcinoma, the development of Paget Disease is yet to be reported. Should Pagetoid cells be observed histologically, Toker cell hyperplasia — a benign condition — must be considered. Mucin and immunohistochemical stains will differentiate the two.
Our expert’s viewpoint
Ginette A. Okoye, MD, FAAD
Professor & Chair
Howard University College of Medicine
Mammillary fistula (MF) is likely a manifestation of hidradenitis suppurativa (HS). The demographics, anatomic location, and association with smoking and squamous cell carcinoma (SCC) support this assertion. Although submammary abscesses and sinus tracts are common in HS, involvement of the mammary tissue, especially in the sub-areola area, is less common and should be alarming to the patient and provider. I have several patients with HS and mamillary fistulae. These lesions are not subtle: my patients have sub-areola nodules and/or induration, inverted nipples, overlying peau d’orange, and purulent drainage from the nipple or areola. I refer these patients for urgent mammograms, and I send them to a breast surgeon for excision, if needed. Unfortunately, recurrence is common after excision, especially if the patient is not on concurrent anti-inflammatory therapy. Additionally, the excisions often result in scarring and asymmetry of the breasts, and exacerbation of the dysmorphia that plagues patients living with HS. My ongoing concern is that we could miss a diagnosis of breast cancer in patients with MF given their distorted anatomy, scar tissue, and blunting of our response to the recurrent ‘false alarms’ seen on their breast exams.
Additionally, Dr. Heymann’s commentary highlights the gap in our understanding of the histopathology of hidradenitis suppurativa. Some of the histologic features of HS overlap with SCC (7) and (apparently!) Paget’s disease, so building our expertise in the histopathology of HS has important implications. Patients with HS are increasingly undergoing en bloc excisions and de-roofing procedures. I strongly advocate for IRB-approved prospective collection of the discarded tissue from these procedures to help build HS tissue biorepositories. The availability of this tissue (and the associated clinical information) will facilitate observations and research to improve our understanding of this terrible disease. (8)
Torre-Castro J, Haya-Martínez L, Ruffin-Vicente B, Moya-Martínez C, Núñez-Hipólito L, Díaz de la Pinta J, Cullen-Aravena D, Jo-Velasco M, Requena L. Toker cell hyperplasia in Zuska disease: A tricky association. J Cutan Pathol. 2021 Jan;48(1):180-183.
Zuska JJ, Crile G Jr, Ayres WW. Fistulas of lactifierous ducts. Am J Surg. 1951 Mar;81(3):312-7.
Huws AM, Semkin L, Moalla A, Udayasankar S, Holt SDH, Sharaiha YM. Primary squamous cell carcinoma of the breast in association with Zuska's disease. Breast Cancer. 2018 May;25(3):365-369.
Cosman BC, Al-Refaie WB. Mammillary fistula as a manifestation of acne inversa (hidradenitis suppurativa): report of two cases. J Am Coll Surg. 2002 Jun;194(6):829-33.
Torous VF, Schnitt SJ, Collins LC. Benign breast lesions that mimic malignancy. Pathology. 2017 Feb;49(2):181-196.
Chapman S, Delgadillo D III, Barber C, Khachemoune A. Cutaneous squamous cell carcinoma complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Mar;27(1):25-28.
Dunstan RW et al. Histologic progression of acne inversa/hidradenitis suppurativa: implications for future investigations and therapeutic intervention. Exp Dermatol 2021;30:820-830. DOI 10.1111/exd.14273.
Byrd AS, Dina Y, Okoh UJ, Quartey QQ, Carmona-Rivera C, Williams DW, et al. Specimen collection for translational studies in hidradenitis suppurativa. Sci Rep. 2019 Aug 21;9(1):12207. DOI: 10.1038/s41598-019-48226-w
All content found on Dermatology World Insights and Inquiries, including: text, images, video, audio, or other formats, were created for informational purposes only. The content represents the opinions of the authors and should not be interpreted as the official AAD position on any topic addressed. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment.
DW Insights and Inquiries archive
Explore hundreds of Dermatology World Insights and Inquiries articles by clinical area, specific condition, or medical journal source.
All content solely developed by the American Academy of Dermatology
The American Academy of Dermatology gratefully acknowledges the support from Incyte Dermatology.
 Make it easy for patients to find you.
Make it easy for patients to find you.
 Meet the new AAD
Meet the new AAD
 2022 AAD VMX
2022 AAD VMX
 AAD Learning Center
AAD Learning Center
 Need coding help?
Need coding help?
 Reduce burdens
Reduce burdens
 Clinical guidelines
Clinical guidelines
 Why use AAD measures?
Why use AAD measures?
 Latest news
Latest news
 New insights
New insights
 Combat burnout
Combat burnout
 Joining or selling a practice?
Joining or selling a practice?
 Advocacy priorities
Advocacy priorities
 Promote the specialty
Promote the specialty

