2022 Innovation Academy: First-time exhibitor information

Reserve your booth space today and join us for the 2022 AAD Innovation Academy!
We are excited to offer your company the opportunity to reconnect in-person with dermatology experts and decision makers. To allow your team to focus on building brand awareness, we want to reassure you our priority is delivering a meeting that puts the health and safety of our attendees and exhibitors first. Safety protocols recommended by the CDC will be followed. The Vancouver Convention Center has been a leader in adopting COVID-19 precautions and is a Global Biorisk Advisory Council® (GBAC) STAR™ accredited facility. Visit aad.org/IA22 for the most up-to-date information.
New applicant submissions
The American Academy of Dermatology invites you to participate in the 2022 AAD Innovation Academy. Take advantage of meeting with the most dedicated dermatology experts and decision makers from around the world.
Generate leads that will increase sales.
Develop advantageous relationships to further expand your business.
Check out the marketplace and gain insight on emerging trends.
Applicants who have either never exhibited at an Academy meeting or have not exhibited in the past 3 years must complete the review process, prior to assignment of exhibit space. An applicant who has exhibited must complete the review process only if there has been a material change in circumstances relating to its company (e.g., a change in ownership, control, or legal status) or in the nature, name, composition, products, labeling, or regulatory status of the products and services to be exhibited.
Company review requirements
Space Application/Contract.
Company profile. The information should include a copy of the company’s filed Articles of Incorporation or W9, company history, mission statement, management team bios, and advisory/board of directors listing, if applicable.
The products and/or services the company plans to exhibit (i.e. product brochures or literature).
Documentation of FDA filing status (if applicable) or acknowledgement of compliance with FDA policies.
Exhibitors must disclose details on any consumer or government litigation, orders, injunctions, judgments, or settlements over the last three years regarding the business practices of the company or the products and services to be exhibited. Companies with multiple complaints filed against them with state or federal consumer affairs regulatory agencies, the Better Business Bureau, or Academy members may be required to provide an explanation of the resolution of those complaints.
First-time exhibitor resources
Space application (to email along with required company information)
Questions?
Please contact Exhibits at exhibits@aad.org.
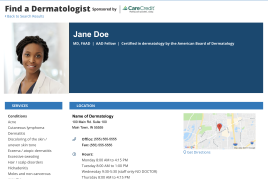 Make it easy for patients to find you.
Make it easy for patients to find you.
 Meet the new AAD
Meet the new AAD
 2022 AAD VMX
2022 AAD VMX
 AAD Learning Center
AAD Learning Center
 Need coding help?
Need coding help?
 Reduce burdens
Reduce burdens
 Clinical guidelines
Clinical guidelines
 Why use AAD measures?
Why use AAD measures?
 Latest news
Latest news
 New insights
New insights
 Combat burnout
Combat burnout
 Joining or selling a practice?
Joining or selling a practice?
 Advocacy priorities
Advocacy priorities
 Promote the specialty
Promote the specialty
